28 Feb 2022 The antioxidant debate in sport and recovery
Should we or should we not supplement hard training athletes with antioxidants, and in what form?
Ian Craig, Registered Nutritional Therapy Practitioner and Founder of the Centre for Integrative Sports Nutrition, explores the evidence on dietary and supplementary antioxidants.
The sales value of over-the-counter vitamins and minerals in Great Britain was over 431.5 million British pounds in 2019, an increase of 2.2% on the previous year (statistica.com). One key market for these dietary supplements is in athletes. Dietary supplements are widely used in the field of sports nutrition to support athletes in all aspects of performance from training, competition, and recovery. Elite athletes recognise the central role nutrition plays in performance, with Nutritionists forming an integral part of most teams. Beyond this world of elite athletes there are however millions of individuals involved in sport and fitness, all of whom are increasingly familiar and regular users of dietary supplements. Antioxidant support is typically top of the list but what are the key considerations to supplementing versus enriching your diet with antioxidant ingredients?
We have all been taught that dietary antioxidants and endogenous antioxidant systems are of fundamental importance in the protection of cellular, mitochondrial, and vascular membranes, genetic molecules, and in the modulation of inflammation. Conversely, exposure of our body to chronic patterns of oxidative stress, that outstrip our antioxidant buffering capabilities, has been associated with most diseases and accelerated ageing. Presented in this way, it is a simple case of “good” and “bad”: it’s “good” to support our antioxidant systems and “bad” to ignore anything that may compromise their capacity.
From this backdrop, it may seem pertinent to advocate the liberal use of exogenous antioxidant nutrients for health. However, whenever anything sounds too straight forward in physiological terms, it often is… Every single physiological process in our body, even if it could be labelled as “bad”, is there for an important reason. And that one piece of information is fundamental to the way we should think about our body: instead of asking “how do I combat this process with intervention strategies”, start by considering the question; “why is this process there in the first place?” In the past decade or two, scientists have realised that reactive oxygen species (ROS), together with reactive nitrogen species (RNS), present a central role in tissue repair (1). What’s more, mitochondria are considered the main source of ROS production in our cells: in this context, ROS act as signalling molecules, which moderate redox regulation of cell proliferation, differentiation, and apoptosis, plus very importantly, mitochondrial biogenesis (2).
Oxidative stress in an exercising context
If we switch over to the intended context of this article, that of exercise training, we can now progress through a similar line of reasoning.
Turning the clock back about 15 years, the thinking then was that exercise created a heightened state of oxidative stress, and that antioxidant supplements may be desirable to quench that physiological response, thereby protecting tissues from damage, and expediting recovery between training bouts.
When I lecture on this topic, I start with a slide entitled ‘Vit C can reduce DOMS’; DOMS stands for Delayed Onset Muscle Soreness, the stiffness/soreness that occurs in response to unfamiliar or excessive exercise. In 2006, Bryer and Goldfarb put 18 healthy men through a set of 70 eccentric elbow extensions with their non-dominant arm. Half the group consumed three grams of vitamin C per day for three weeks before the trial, whereas the other half took a placebo. It was shown that the inclusion of vitamin C diminished muscle soreness in the first 24 hours, plus attenuated the rise in creatine kinase (an indicator of cellular damage) and oxidised glutathione levels (3).
However, the very next slide in my presentation is entitled ‘Vit C may prolong DOMS!’ because around the same time other researchers were finding equivocal or detrimental results of vitamin C supplementation. Graeme Close and colleagues supplemented subjects with one gram per day of vitamin C (or placebo) for two days before and 14 days after a downhill running bout. They noted that vitamin C supplementation attenuated oxidative stress, but in the process also delayed the recovery of muscle function (4).
In line with the later observation, it has been proposed by countless researchers that reactive oxygen species, created during exercise, are a crucial signal for initiation of the repair process via the activation of redox-sensitive signalling pathways (5-11). As such, dietary antioxidant supplementation may in fact suppress skeletal muscle repair, not aid it. A case in point was demonstrated by Gomez-Cabrera et al., who trained 14 men for eight weeks while supplementing one gram of vitamin C per day or placebo (5). They found that long-term, in their words “excessive”, administration of vitamin C hampered skeletal muscle adaptation and endurance capacity of the subjects, possibly due to an attenuated exercise-induced increase in certain key transcription factors for mitochondrial biogenesis.
Very recently, Scott Powers and colleagues extended this notion of oxidative stress as a signal for adaptive responses into the realm of muscular propagation, by explaining that NFκB (nuclear factor kappa B) is also involved in oxidative stress induced signalling, and that skeletal muscle adaptations don’t just include mitochondrial biogenesis, but also muscle hypertrophy (9). Their overall research observations led to Powers et al. making the following comment: “Hence, it appears that contraction-induced ROS production is a key signalling molecule in resistance training-induced muscle hypertrophy.”
Due to these lines of thought (that oxidative and inflammatory responses are required for musculoskeletal adaptions to occur), many researchers have cautioned against the use of high-dose antioxidant supplementation in an exercise context. Kurutas even went one step further and suggested that upsetting the cell signalling pathways may increase the risk of chronic disease (8).
However, results over the years have been equivocal: for example, Yfanti and colleagues found no dampening of exercise adaptations with vitamin C and E supplementation (11), while Powers and Jackson demonstrated a delay in muscular fatigue with N-acetylcysteine supplementation (12). The comprehensive review by He et al. also noted reduced muscular fatigue and enhanced exercise recovery with antioxidant supplementation (13). Therefore, the picture is not quite as clear as a “yes” (supplement) or “no” (don’t supplement) scenario.
Let’s add individuality into the mix…
Critically, and very sensibly, He et al. focussed on the individual athlete in question, suggesting that the physiological effect of antioxidant supplementation will depend on their baseline redox status, the dose and duration of supplementation, and the oxidative stress markers measured (13). This notion has been supported by other researchers, including Kawamura and Muraoka, who have observed a wide inter-individual variability in redox status amongst athletes; at rest and during acute exercise (14). They suggested that these variable responses may provide important clues about why such study results are inconsistent.
Exercise immunologist Neil Walsh also took a balanced approach to the use of antioxidants in his recent review: he noted that vitamin C is a cheap and safe supplement and has been shown to prevent upper respiratory tract symptoms during heavy training, so he suggested that athletes consider making use of vitamin C supplementation during periods of heightened infection risk (15).
Kurutas also conferred that vitamin C can create antioxidant and pro-oxidant effects, depending upon the dose and redox status of the individual (8), noting that this is also the case for other antioxidants, including carotenoids, vitamin E and alpha lipoic acid.
A balanced philosophical perspective
If we step away from the confines of exacting research measurements for a moment, we can access a wider view perspective of this fascinating debate. If certain researchers are correct in their assertion that antioxidant supplementation can inhibit the adaptive responses to exercise, the tempting thing to do (in our modern yes/no way of thinking) is to completely avoid antioxidant supplementation around exercise. However, let’s take a worst-case scenario in this regard: an athlete, who eats scant amounts of fruits and vegetables, trains hard twice a day, doesn’t sleep enough, and is exposed daily to environmental toxicity in the form of second-hand cigarette smoke. It should be clear that antioxidant depletion is more likely than antioxidant nourishment in this individual, meaning that his or her baseline redox status would favour pro-oxidation. For nutrition practitioners, our first approach should be to educate this athlete on the important merits of a balanced diet for example, adequate fruit and vegetable consumption. However, he or she may not achieve the BANT Wellbeing Guidelines 7-per-day recommendations, and even so, it might not be sufficient to balance out their high training and toxicity-induced antioxidant demands. Additionally, perhaps this athlete also possesses single nucleotide polymorphisms (SNPs) on certain detoxification and oxidative stress-oriented genes.
All these (physiologically stressing) factors could predispose our athlete to mitochondrial membrane and genome dysfunction and mitophagy (16,17), longer term myocardial scarring and cardiac arrhythmias (18), plus many other disease states. Or, as Simioni et al. said; “chronic exposure to high levels of ROS can become toxic, exhausting the enzymatic and non-enzymatic antioxidant system, and leading to impaired cellular function, macromolecule damage, apoptosis, and necrosis (19).” In other words, in an N = 1 scenario, this athlete may greatly benefit from a very focussed antioxidant-supporting food and supplement strategy, such as vitamins A, C and E, Coenzyme Q10, catechins in green tea, resveratrol in red wine, lycopene in tomatoes, and many others (9,12,20).
On the other hand, Margaritelis et al., who after studying the oxidative stress responses of 98 young men to an acute bout of eccentric (muscle damaging) exercise, concluded; “although exercise induces oxidative stress in the majority of individuals, it can induce reductive stress or negligible stress in a considerable number of people (21).” Perhaps this is why some individuals survive multiple years of intensive training and competition relatively unscathed, whereas others noticeably age before our eyes. Hopefully, in time, researchers will consider correlating the data that can now be obtained from nutrigenetic testing with other health parameters that associate with longevity in long term serious exercisers.

Exercise-induced antioxidant adaptation
It may be tempting to step back from all these oxidative stress observations and say, “I knew exercise was bad for us”, and promptly demote your activity levels to that of armchair football. However, it is critical to understand that although acute exercise can, in some cases, drive oxidative stress to high levels, the right amount of chronic exercise (in context of an individual’s life) can promote radical activation of our antioxidant enzyme systems.
According to Simioni et al., excessive training can be physiologically detrimental to untrained individuals (at first), but that exercise training of a progressive nature could help the active cells to adapt to detoxifying a large ROS load (19). Similarly to what we’ve heard before, Kawamura and Muraoka added that; “exercise intensity and duration, nutritional intake, and training status are important factors that affect exercise-induced oxidative stress (14).”
If we look at training studies, a plethora of research, over at least four decades, has studied the effects of exercise on oxidative stress in athletes. For example, Viguie and colleagues asked 11 moderately trained young men (average age 24) to cycle for 90 minutes at a moderate intensity of 65 per cent VO2peak on three consecutive days (22). On each day, oxidised glutathione levels increased, and ascorbate (vitamin C) levels decreased significantly within the first 15 minutes of exercise, but levels returned to normal within 15 minutes of recovery, meaning that these were simply transient responses. Interestingly, it has been shown that as few as five to ten consecutive days of endurance exercise can create substantial increases in the antioxidant capacity of skeletal muscle fibres (9).
A hormetic perspective on oxidative stress
The oxidative stress ‘needs’ of the body for health and physical performance adaptations, in the right dose, is a really good example of hormesis. Hormesis is a term used in biology to describe a biphasic dose–response curve, whereby a transient low dose of a stressor initiates a beneficial cellular adaptive response, and a chronic and/or high dose of the stressor results in cellular damage. As shown in Figure 1, the bell-shaped hormesis curve proposed by Powers et al (9), suggests that increasing levels of exercise-induced ROS will stimulate positive physiological adaptations until the point where oxidative stress starts to out-weigh antioxidant capacity of the body, when further exercise can only create cellular damage, inhibit adaptations to exercise, and potentially curtail an athletic career and expedite ageing.
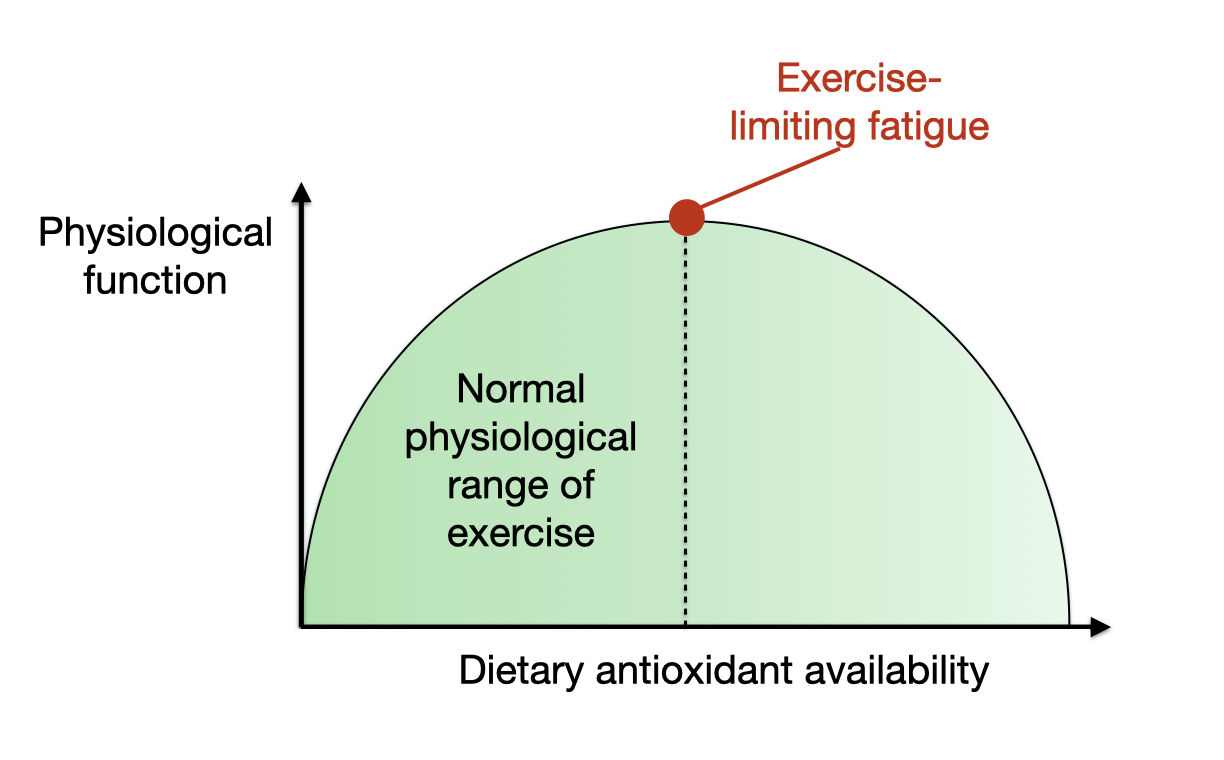
Figure 1 – Bell shaped model of exercise stress and physiological function
It is true that exercise is essential for our whole-body health in general terms, but within a personalised way of working with athletes, it is pertinent that we identify when they have tipped to the right-hand side of the hormesis model. In practise, myself and other functional practitioners do see many extreme natured athletes who have tipped to the negative side of the double-edged sword of ROS production and are now suffering from a multitude of ills. Their body will certainly defend (to the best of its ability) against damage during short term stressors, but if the levels of oxidative stress exceed that of our buffering antioxidants for multiple years, the outcome may be very different.
This tipping point between health and ill-health is heavily influenced by what else is happening in our athletes’ lives. In real terms most athletes train for an allocated hour or two each day. This leaves up to 22 hours of each day in which they are not training. For practitioners, our focus should be on making this period as recuperative and regenerative as possible.
A food-first approach
To finish this article, it gives me great pleasure to move from the academic playground, where researchers seek definitive answers to questions like these, to the majestic ecosystem of nature. For a long time, it has made intuitive sense to me to supply athletes with foods that are stacked with antioxidants: dark, colourful fruits and vegetables, especially organic varieties or wild gathered. As such, my suggested DIY sports drinks are based on fruit juices including grape and pomegranate, and teas including green varieties and rooibos (red bush). My antioxidant recovery smoothies include berries (especially wild brambles/blackberries) and often a powered extract of a superfood or two. In this way, my athletes are including antioxidants in their training strategy, but in a ‘nature’s balance’ kind of way. I also make use of more direct antioxidant nutrient supplements on a case-by-case basis as per the one-size-fits-one model of personalised nutrition and lifestyle medicine. This way of working has coincided with an exciting era within sports nutrition where we are seeing an acceleration in research on food compounds.
Thirteen years ago, at the University of Exeter, Andy Jones and colleagues conducted tests to assess the effect of regular consumption of beetroot juice compared to blackcurrant cordial on bicycle time to exhaustion (23). On average, subjects cycled for 16 per cent longer after consuming ½ litre of the beetroot juice per day for six days. Because a food (in this case beetroot) showed a clear ergogenic advantage, suddenly the whole sporting fraternity started studying fruits and vegetables in this performance context. We since seen research with positive outcomes on tart cherries (24), sugar cane juice (25), acai berries (26), watermelon (27), New Zealand blackcurrants (28), dark chocolate (29,30), and many others.
If, as practitioners, we had access to expensive antioxidant and oxidative stress testing, we could create very personalised antioxidant supplementation strategies on an individual basis. Failing that scenario, however, we can still tap into the power of food for our antioxidant supplies with evidence-based personalised nutrition recommendations. It’s not just about singular food extracts as the above research might suggest. Too much of any singular ingredient can potentially tip the balance e.g., we don’t know what would happen to our adaptive responses after a year of consuming ½ litre of beetroot juice per day. However, if we use this information in recommendations which encourage daily consumption of a wide variety of colourful foods, including those shown to have beneficial antioxidant effects in the research, we can most certainly support our athletes’ heath-based-performance in a positive and nourishing way.

Example of an antioxidant-rich recovery smoothie
• 50-100ml natural whole yoghurt (just ‘milk and cultures’ on ingredients list) or 50-100ml kefir
• 50-100ml freshly squeezed fruit juice (grape, berry, apple)
• ½ – 1 cup fresh or frozen berries (especially wild-gathered, such as brambles/blackberries) equiv. 80-150g
• 1 banana
• 20-30g plain grass-fed organic whey protein (or egg, hemp, brown rice, or pea etc.)
• Superfood powder – eg. 1-2 tsp baobab, camu camu, mesquite etc.
BLEND ALL INGREDIENTS TOGETHER AND DRINK IMMEDIATELY
Additional BANT Resources
BANT Wellbeing Guidelines:
https://bant.org.uk/bant-wellbeing-guidelines/
BANT Eat a Rainbow Infographic:
https://bant.org.uk/foodforyourhealth_tools/infographics/
Nutrition Evidence InfoBite on Antioxidants in athletes:
https://bant.org.uk/foodforyourhealth_tools/ned-info-bites/
About Ian Craig
 Ian Craig MSc DipCNE BANT INLPTA is an experienced exercise physiologist, nutritional therapist, NLP practitioner and an endurance coach. He is the founder of the Centre for Integrative Sports Nutrition and course leader for the online Certificate of Integrative Sports Nutrition course, plus he teaches on the postgraduate Personalised Sports Nutrition course at CNELM. Clinically, within a team dynamic, Ian works with sporting individuals and complex health cases at his Scottish home, and online. Additionally, Ian is the editor of Functional Sports Nutrition magazine and he co-authored the Struik Lifestyle book Wholesome Nutrition with his natural chef wife Rachel Jesson. www.intsportsnutrition.com; Twitter @ian_nutrition; FB
Ian Craig MSc DipCNE BANT INLPTA is an experienced exercise physiologist, nutritional therapist, NLP practitioner and an endurance coach. He is the founder of the Centre for Integrative Sports Nutrition and course leader for the online Certificate of Integrative Sports Nutrition course, plus he teaches on the postgraduate Personalised Sports Nutrition course at CNELM. Clinically, within a team dynamic, Ian works with sporting individuals and complex health cases at his Scottish home, and online. Additionally, Ian is the editor of Functional Sports Nutrition magazine and he co-authored the Struik Lifestyle book Wholesome Nutrition with his natural chef wife Rachel Jesson. www.intsportsnutrition.com; Twitter @ian_nutrition; FB
References
- Gonçalves RV, Costa AMA, Grzeskowiak L. Oxidative stress and tissue repair: mechanism, biomarkers, and therapeutics. Oxid Med Cell Longev. 2021 Feb 27;2021:6204096.
- Yoboue ED, Devin A. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int J Cell Biol. 2012 May 30;2012:403870.
- Bryer SC, Goldfarb AH. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int J Sport Nutr Exerc Metab. 2006 Jun;16(3):270-80.
- Close GL, Ashton T, Cable T, Doran D, et al. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006 May;95(5):976-81.
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008 Jan;87(1):142-9.
- Horn A, Van der Meulen JH, Defour A, Hogarth M, et al. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci Signal. 2017 Sep 5;10(495):eaaj1978.
- Jackson MJ. Free radicals generated by contracting muscle: by-products of metabolism or key regulators of muscle function? Free Radic Biol Med. 2008 Jan 15;44(2):132-41.
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016 Jul 25;15(1):71.
- Powers SK, Deminice R, Ozdemir M, Yoshihara T, et al. Exercise-induced oxidative stress: Friend or foe? J Sport Health Sci. 2020 Sep;9(5):415-25.
- Webb R, Hughes MG, Thomas AW, Morris K. The ability of exercise-associated oxidative stress to trigger redox-sensitive signalling responses. Antioxidants (Basel). 2017 Aug 10;6(3):63.
- Yfanti C, Akerström T, Nielsen S, Nielsen AR, et al. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc. 2010 Jul;42(7):1388-95.
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008 Oct;88(4):1243-76.
- He F, Li J, Liu Z, Chuang CC, et al. Redox mechanism of reactive oxygen species in Front Physiol. 2016 Nov 7;7:486.
- Kawamura T, Muraoka I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants (Basel). 2018;7(9):119.
- Walsh NP. Nutrition and athlete immune health: New perspectives on an old paradigm. Sports Med. 2019 Dec;49(Suppl 2):153-68.
- Müller-Höcker J. Mitochondria and ageing. Brain Pathol. 1992 Apr;2(2):149-58.
- Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69(18):2999-3013.
- O’Keefe JH, Patil HR, Lavie CJ, Magalski A, et al. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc. 2012 Jun;87(6):587-95.
- Simioni C, Zauli G, Martelli AM, Vitale M, et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018 Mar 30;9(24):17181-98.
- Knez WL, Coombes JS, Jenkins DG. Ultra-endurance exercise and oxidative damage : implications for cardiovascular health. Sports Med. 2006;36(5):429-41.
- Margaritelis NV, Kyparos A, Paschalis V, Theodorou AA, et al. Reductive stress after exercise: The issue of redox individuality. Redox Biol. 2014 Feb 19;2:520-8.
- Viguie CA, Frei B, Shigenaga MK, Ames BN, et al. Antioxidant status and indexes of oxidative stress during consecutive days of exercise. J Appl Physiol (1985). 1993 Aug;75(2):566-72.
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985). 2009 Oct;107(4):1144-55.
- Bowtell JL, Sumners DP, Dyer A, Fox P, et al. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc. 2011 Aug;43(8):1544-51.
- Kalpana K, Lal PR, Kusuma DL, Khanna GL. The effects of ingestion of sugarcane juice and commercial sports drinks on cycling performance of athletes in comparison to plain water. Asian J Sports Med. 2013 Sep;4(3):181-9.
- Sadowska-Krępa E, Kłapcińska B, Podgórski T, Szade B, et al. Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol Sport. 2015 Jun;32(2):161-8.
- Shanely RA, Nieman DC, Perkins-Veazie P, Henson DA, et al. Comparison of watermelon and carbohydrate beverage on exercise-induced alterations in systemic inflammation, immune dysfunction, and plasma antioxidant Nutrients. 2016 Aug 22;8(8):518.
- Braakhuis AJ, Somerville VX, Hurst RD. The effect of New Zealand blackcurrant on sport performance and related biomarkers: a systematic review and meta-analysis. J Int Soc Sports Nutr. 2020 May 27;17(1):25.
- Patel RK, Brouner J, Spendiff O. Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. J Int Soc Sports Nutr. 2015 Dec 15;12:47.
- Allgrove J, Farrell E, Gleeson M, Williamson G, et al. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int J Sport Nutr Exerc Metab. 2011 Apr;21(2):113-23.