15 Apr 2024 Getting to grips with Gut Health: The doom of Dysbiosis – Part 2
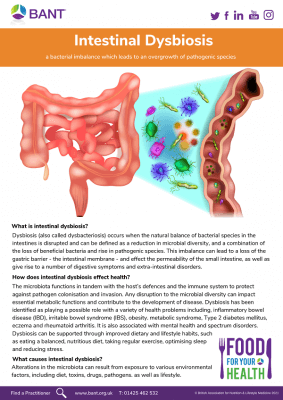
The doom of dysbiosis: an imbalance of your gut microbiota
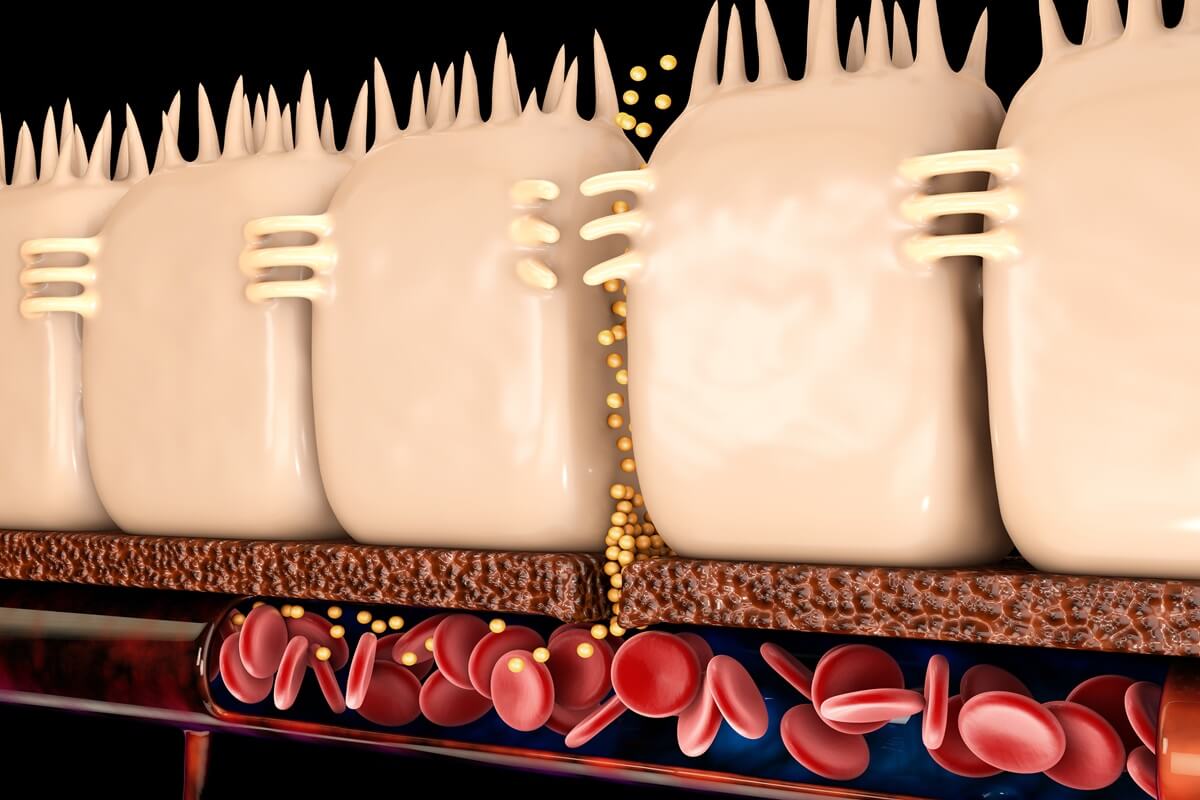
We know by now that dietary habits can have a powerful impact on the composition of our microbiota. Sadly, the conventional Western-style diet that is so common the UK is overly processed and packed with sugar, salt, fat and additives which can reduce gut microbial diversity and lead to dysbiosis. [1][2]
Dysbiosis simply indicates an imbalance and instability of the gut microbiota which can lead to a variety of adverse health problems. This can create a snowball effect, altering our gut barrier function and permeability, and abnormally activating our immune cells, leading to high incidences of chronic diseases across the body.[3]
When our immune systems go into overdrive and begin to attack the gut because of dysbiosis and a microbiome lacking in diversity and volume, we can develop serious and debilitating conditions such as IBD and IBS. IBD is often treated using immunosuppressant drugs which dampens the immune system to decrease inflammation. But there are consequences. Antibiotics are also frequently used and widely known to wipe out all gut bacteria, thereby removing both pathogenic and beneficial bacteria that had once colonised the gut.
A review of 19 different studies involving 2,609 patients with IBD found that high intakes of total fats, polyunsaturated fatty acids, omega-6 fatty acids, and meat were routinely linked with an increased risk of ulcerative colitis and Crohn’s disease. In contrast, high vegetable consumption was consistently associated with a decreased risk of ulcerative colitis, while fibre and fruit intake was linked with a reduced risk of Crohn’s disease.[4]
Another study of 67,581 women between the ages of 40 and 65 in France, showed that high-protein, high-fat diets rich in red meat were strongly linked to the onset of IBD.[5]
The gut microbiome has a vital role in directing immune system development. Dysbiosis appears to go hand-in-hand with many inflammatory diseases of the gastrointestinal tract although researchers are still investigating whether these conditions gut imbalances and dysbiosis, or if dysbiosis contributes to the development of these diseases.[6]
Below we outline three common gastrointestinal disorders, highlighting their symptoms, treatment and dietary recommendations.
Common gastrointestinal disorders
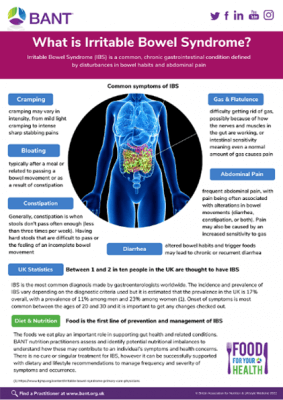
Irritable bowel syndrome (IBS) is marked by recurrent abdominal pain and discomfort associated with altered bowel habits. Key symptoms of IBS include diarrhoea or constipation, abdominal pain, cramping, bloating and changes in stool pattern. [7]
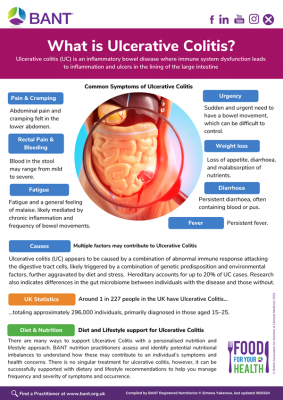
Inflammatory bowel diseases (IBD) are a group of autoimmune disorders that include Crohn’s disease and ulcerative colitis. Hallmark characteristics of IBD are inflammation of both the small and large intestine, where parts of the digestive system are attacked by the body’s own immune system.[8]
Coeliac Disease is also an autoimmune condition in which people with a genetic predisposition have an intolerance to gluten.[9]
| Condition | Common signs & symptoms | Conventional treatment | Dietary intervention |
| Irritable bowel syndrome
Four forms – IBS – Constipation, IBS – Diarrhoea IBS – Mixed IBS – Unclassified |
Abdominal pain
Constipation or diarrhoea Changes in stool pattern Mucus and/or blood in the stools Cramping Pain Vomiting Nausea Flatulence Bloating[10] |
Antispasmodics
Peppermint oil Tricyclic anti-depressants Loperamide for diarrhoea Laxatives for constipation Cognitive behavioural therapy Gut-directed hypnotherapy[11] |
Low FODMAP diet
Probiotics Limiting alcohol and caffeine |
| Inflammatory bowel disease
Includes Crohn’s disease and ulcerative colitis |
Chronic intestinal inflammation
Abdominal pain Diarrhoea, sometimes with blood Urgency to pass a stool and fecal incontinence Rectal bleeding Weight loss Fever Anaemia Malnutrition and delayed growth in those who have IBD as children Anxiety and depression[12] |
Immunosuppressive drugs to modulate an overactive immune system
Anti-inflammatory drugs to dampen inflammation[13] |
Mediterranean diet (with guidance on fibre to avoid exacerbating gut inflammation)
Plant-based diet Low FODMAP diet Removal or major reduction of red meat and other animal proteins[14] Probiotics (but not for Crohn’s)[15] |
| Coeliac disease | Symptoms can vary significantly.
Abdominal pain Bulky, pale, foul smelling, greasy stools Weight loss and signs of vitamin and mineral deficiencies |
Strict permanent gluten-free diet. | Strict permanent gluten-free diet. Removal of wheat including durum, semolina, spelt, kamut, einkorn, and farro. Removal of rye, barley and oats (unless oats are certified gluten-free).
Buckwheat and millet are often excluded as well |
Dysbiosis and disease
The negative effects of a disturbed microbiome can move far beyond digestive disorders, wreaking havoc in other systems of the body.
Mental health disorders
Research on the gut-brain axis is growing exponentially, with mounting evidence showing that what we eat can have a direct and profound impact on our mental health and on chronic neurological conditions.[16][17]
The gut-brain axis comprises a complex bi-directional relationship involving signals from the nervous, endocrine and immune systems. It links our central nervous system with the enteric nervous system that lives in our gut, thereby linking emotional and cognitive centres of the brain with intestinal functions.
Many neurotransmitters are made in the gut including around 95% of the body’s serotonin[18], along with dopamine, norepinephrine and noradrenaline for focus, and GABA for relaxation. What we consume could potentially have a profound effect on neurotransmitter production and mental health conditions. This is particularly in light of the fact that bacteria themselves have the ability to produce and influence levels of neurotransmitters.[19]
Abundant evidence now suggests that people with mental health disorders typically consume an excess of high fat and high sugar foods alongside inadequate intake of nutrient dense foods compared to the general population.
A systematic review of 18 articles found a clear link between the composition of gut microbiota and depression, stress, autism spectrum disorder (ASD), psychotic episodes, eating disorders, anxiety and brain function.[20] This serves to illustrate further the importance of maintaining a healthy and nutritious diet.
Cancer
How can gut microbiota imbalances lead to cancer? What are the mechanisms? It is hard to draw a direct correlation, but researchers have pointed to the immunomodulatory effects of microbiota which can have profound implications for tumour growth, surveillance and metastases.[21] In essence, the microbes in our gut could play an interesting role in helping our immune system fight carcinogenic cells or perhaps slow their growth. More trials are needed to establish how this could work.
In one study in 2020, researchers examined 1,526 tumours and their surrounding tissues across seven types of cancer: lung, breast, pancreas, ovary, bone, brain and melanoma. They found that each tumour type had its own distinct microbiome composition with breast cancer tumours showing particularly diverse and rich microbiota.[22]
Evidence such as this does not indicate a direct causal relationship between the tumour microbiome and the development of cancer. However, it is propelling scientists to explore how microbiota may be examined to diagnose specific cancers, and how the microbiome could be potentially altered for therapeutic purposes in cancer care.[23]
Best practices
What can you do beyond cultivating good eating habits to boost your microbiome?
Probiotics
The wellness industry has propelled a number of supplements, elixirs and specialty foods into the marketplace, promising consumers a quick fix for gut-related ailments. Like some of these products, probiotics are increasingly hailed as a “cure-all” for gut disorders.
In certain cases, for example, to help replenish gut flora following a course of antibiotics, or as a treatment option for IBS and IBD (aside from Crohn’s), probiotics are fundamental to gut recovery. Antibiotics have a profound impact on our microbiome makeup, reducing both the diversity and richness of the community.[24][25] Consistent antibiotic use can lead to antibiotic resistant pathogens which could cause antibiotic-associated diarrhoea and colonic infections, creating a ripe environment for the development of inflammatory bowel disease.[26]
But for those without debilitating gastrointestinal conditions, there are limitations to what probiotics can achieve without habitual dietary consumption of gut-loving foods. While there is a temptation to pop a pill and consider your gut cared for, we all know by now how much we can nurture our microbiome with real food. Consult a nutritionist to help you choose the right probiotic strains, and add that to a plant-rich nutrient-dense diet for more effective and long-lasting results.
Protein
Throughout this piece, we have emphasized the importance of plant-based fibre and polyphenols, complex carbohydrates, essential fatty acids and fermented foods for gut health.
This is not to suggest that we shouldn’t be prioritising protein in our diets. Both animal and vegetarian sources of protein contribute to microbial diversity in different ways. However, high animal protein intake has been associated with a significantly increased risk of inflammatory bowel disease[27][28] and cardiovascular risk, and does not increase the production of short-chain fatty acids the way that vegetarian sources of protein are able to do.[29] So consider minimizing meat intake, sourcing well-reared grass-fed animals, and opting for leaner cuts.
Elimination diets
Throughout the UK and around the world, communities adhere to different dietary norms based on their religious, cultural, ethical and health persuasions. While this is not hugely problematic, providing that they consume essential macro- and micronutrients, research has shown that heavily restricted diets could create limitations in microbial makeup.[30]
Reduced fibre and resulting constipation could be a consequence of long-term adherence to a ketogenic diet, for example, which prioritises fat intake and emphasizes an avoidance of carbohydrates. A long-term low-carbohydrate diet certainly has implications for optimising the gut microbiome.
A long-term plan to eventually cease a rigid exclusion diet is particularly crucial for those working through chronic illnesses, such as IBS or IBD for example, where a FODMAP diet may be suggested to alleviate symptoms. Specialists agree with this as a starting point, but many emphasize the need to work with doctors and nutritionists to reincorporate once-removed healthy foods (as long as they can be tolerated) so as not to compromise the microbiome. A number of foods that must be avoided on the FODMAP diet, such as onions, garlic, apples and pears, contain prebiotic compounds that can help beneficial gut bacteria thrive.
Functional testing
Those affected by gastrointestinal disorders that are difficult to diagnose can feel saddled with anxiety and depression at the thought of living with unresolved often painful symptoms. Functional testing may help to pinpoint which part of the gut requires support, and help to confirm levels of inflammation. An endoscopy with biopsies of the small intestine and potential cross-section imaging could be useful for those suffering with Crohn’s disease. In the case of IBS, it may be worth testing to rule out pelvic floor disorders in those with chronic constipation, fecal incontinence, overflow diarrhoea or other defecatory disorders since these conditions could respond to biofeedback therapy.[31]
Exercise
Studies show that exercise overall has a positive effect on building microbial diversity and raising butyrate production.[32] In one study involving elite rugby players, exercise was found to enrich microflora diversity. The healthy bacterial presence also correlated positively with protein intake and creatine kinase levels, suggesting that both diet and exercise contributed microbial benefits. In another study, the microbiome of individuals with varying levels of fitness was examined. The results showed that regardless of diet, high cardio-respiratory fitness was aligned with increased microbial diversity.[33]
Environment
Studies have shown that the urbanization of outdoor spaces, a rise in building confinement and cleaning practices could decrease microbial diversity.[34] One could posit then the pronounced implications that the covid pandemic could have exerted on gut microbiome composition in populations around the world – not only because of the hit to our immune systems, and obsessive sanitation, but also because of a heightened period of isolation, confinement and quarantine indoors.
With this in mind, think about spending time outdoors to vary your environment and routine. Take a walk in the park, go hiking, or participate in a gardening project. Increasing your exposure to nature could add another dimension to diversifying your microbiome.[35]
-END-
Vandana Chatlani, BANT Registered Nutritionist ® NT Dip, mBANT, rCNHC
NOTES TO EDITORS:
BANT is the leading professional body for Registered Nutritional Therapy Practitioners in one-to-one clinical practice and a self-regulator for BANT Registered Nutritionists®. BANT members combine a network approach to complex systems, incorporating the latest science from genetic, epigenetic, diet and nutrition research to inform individualised recommendations. BANT oversees the activities, training and Continuing Professional Development (CPD) of its members.
Registered Nutritional Therapists are regulated by the Complementary and Natural Healthcare Council (CNHC) that holds an Accredited Voluntary Register (AVR) for the Professional Standards Authority for Health and Social Care (PSA). A report by the Royal Society for Public Health and the Professional Standards Agency made a key recommendation that AVR practitioners have the authority to make direct NHS referrals, in appropriate cases, to ease the administrative burden on GP surgeries. BANT nutrition practitioners are the key workforce asset to harness 21st century lifestyle medicine to tackle the rising tide of stress-related fatigue, obesity, Type 2 Diabetes, dementia and other chronic diseases.
To find a BANT nutrition practitioner, please click here
BANT WELLBEING GUIDELINES:
The BANT Wellbeing Guidelines are specifically designed to provide clear, easy to understand general information for healthy diet and lifestyle when personalised advice is not available.
BANT launched it’s 2021 Food for your Health campaign to tackle diet-induced obesity and metabolic dysregulation and combat the junk food culture by promoting healthy food and lifestyle choices. We offer a range of free, open-access resources, and have a network of over 3,500 Nutrition Practitioners throughout the UK.
References:
[1] Influence of diet on the gut microbiome and implications for human health, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5385025/
[2] Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis, https://www.mdpi.com/2076-2607/10/1/167
[3] Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6835969/
[4] Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature, https://www.researchgate.net/publication/51020613_Dietary_Intake_and_Risk_of_Developing_
Inflammatory_Bowel_Disease_A_Systematic_Review_of_the_Literature
[5] Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study, https://www.researchgate.net/publication/44590778_Animal_Protein_Intake_and_Risk_of_
Inflammatory_Bowel_Disease_The_E3N_Prospective_Study
[6] Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3448089/
[7] Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10096616/#B1-nutrients-15-01647
[8] Inflammatory bowel disease: clinical aspects and treatments, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4106026/
[9] Celiac disease: a comprehensive current review, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6647104/
[10] Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10096616/#B1-nutrients-15-01647
[11] Best management of irritable bowel syndrome, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8231425/
12 59 Inflammatory Bowel Disease (IBD), https://www.hopkinsmedicine.org/health/conditions-and-diseases/inflammatory-bowel-disease
[13] Inflammatory bowel disease: clinical aspects and treatments, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4106026/
[14] The role of diet in the prevention and treatment of Inflammatory Bowel Diseases, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6502201/
[15] Gut Microbiome: Profound Implications for Diet and Disease, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6682904/
[16] Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8321864/
[17] The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4367209/
[18] The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6469458/
[19] Neurotransmitter modulation by the gut microbiota, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6005194/
[20] Association between gut microbiota and psychiatric disorders: a systematic review, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10435258/
[21] The microbiome and human cancer, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8767999/
[22] The human tumor microbiome is composed of tumor type–specific intracellular bacteria, https://www.science.org/doi/10.1126/science.aay9189
[23] The microbiome and human cancer, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8767999/
[24] Impact of antibiotics on gut microbiome composition and resistome in the first years of life in low- to middle-income countries: A systematic review, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10298773/
[25] Antibiotics as Major Disruptors of Gut Microbiota, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7732679/
[26] Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease, https://pubmed.ncbi.nlm.nih.gov/20700115/
[27] Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study, https://www.researchgate.net/publication/44590778_Animal_Protein_Intake_and_Risk_of_
Inflammatory_Bowel_Disease_The_E3N_Prospective_Study
[28] Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature, https://www.researchgate.net/publication/51020613_Dietary_Intake_and_Risk_of_Developing_
Inflammatory_Bowel_Disease_A_Systematic_Review_of_the_Literature
[29] Influence of diet on the gut microbiome and implications for human health, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5385025/
[30] Gut Microbiome: Profound Implications for Diet and Disease, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6682904/
[31] Functional gastrointestinal symptoms in patients with inflammatory bowel disease: A clinical challenge, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6581193/
[32] Differences in gut microbiota profile between women with active lifestyle and sedentary women, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0171352
[33] Exercise Modifies the Gut Microbiota with Positive Health Effects, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357536/
[34] Urbanization Reduces Transfer of Diverse Environmental Microbiota Indoors, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2018.00084/full
[35] Impact of outdoor nature-related activities on gut microbiota, fecal serotonin, and perceived stress in preschool children: the Play&Grow randomized controlled trial, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7738543/